ISO 13485:2016 Certification Services at Vektors: Ensuring Excellence in Medical Device Quality Management.
Welcome to Vektors, your trusted partner in achieving excellence in Quality Management Systems for Medical Devices. Our ISO 13485:2016 certification services are designed to empower organizations across Mumbai, Maharashtra (INDIA), and Dubai, UAE, ensuring compliance with regulatory requirements and fostering a culture of continuous improvement.
Why Choose ISO 13485:2016 Certification with Vektors?
ISO 13485:2016, focusing on Medical Devices Quality Management Systems, serves as a robust framework for organizations involved in the design, development, production, installation, and delivery of medical devices. By partnering with Vektors, you unlock a range of benefits:
- Demonstrate Regulatory Compliance: Our certification enables you to showcase adherence to regulatory requirements, providing assurance to stakeholders and regulatory bodies.
- Risk Management and Best Practices: Implement best practices for quality and safety, managing risks effectively throughout your processes.
- Process Improvement: Drive efficiency and excellence in your operations by implementing improved processes aligned with ISO 13485:2016 standards.
- Competitive Advantage: Gain a competitive edge in the market by showcasing your commitment to quality and customer satisfaction.
- Customer Confidence: Build trust and confidence among your customers by adhering to international standards for medical device quality.
Key Changes in ISO 13485:2016
- The latest edition of ISO 13485 brings significant enhancements, including:
- Harmonization of regulatory requirements
- Integration of risk management throughout the quality management system
- Increased clarity on validation, verification, and design activities
- Strengthening of supplier control processes
- Emphasis on feedback mechanisms
- Harmonized requirements for software validation across different applications
New Certification Services for Medical Devices
As of November 2020, we proudly announce the launch of new certification services for manufacturers and traders dealing with various medical devices, including but not limited to:
- Disposable Hypodermic Syringes
- Cardiac Stents
- CT Scan Equipment
- Ultrasound Equipment
- Condoms
- and many more…
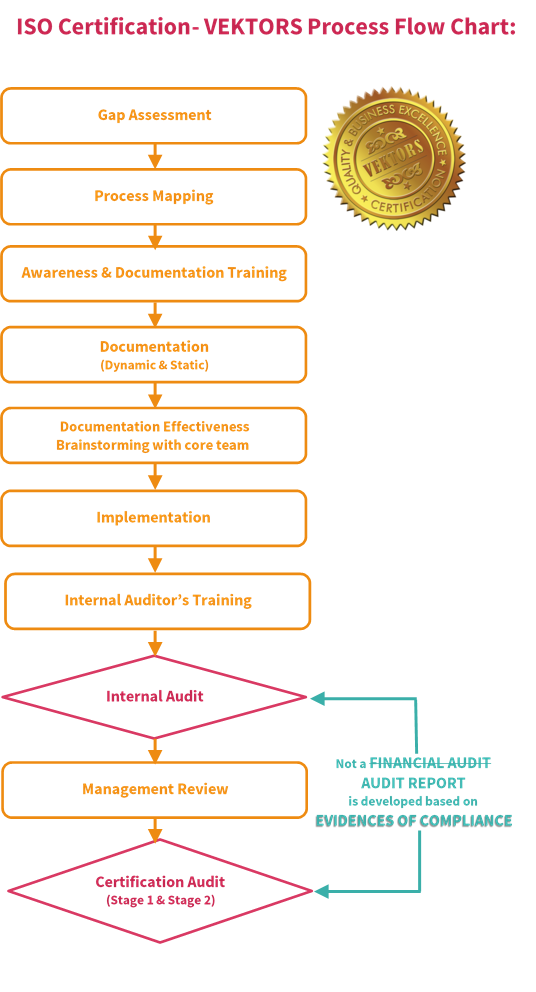
Certification Road-Map and Cost of Certification
Explore our tailored certification services for different categories of medical devices in Mumbai, Maharashtra (INDIA), and Dubai, UAE. Ask for a Certification Road-Map and get insights into the cost of certification.
ISO 13485:2016 Certification Categories
We offer ISO 13485:2016 certification services across a diverse range of medical specialties, including:
Anesthesiology
- Pain Management
- Cardiovascular
- Dental
- Ear, Nose, Throat (ENT)
- Gastroenterological
- and many more…
Our commitment to excellence is reflected in our adherence to the latest version of ISO 13485, ensuring that your medical devices meet the highest standards of quality and compliance. As specialists in ISO Certification for Medical Devices, we guide you through the ISO 13485 requirements, providing expert consultation tailored to your unique needs.
Vektors proudly operates as ISO 13485 Registrars, facilitating a seamless certification process. Our expertise extends to EN 13485, offering comprehensive 13485 Certification services that align with global standards. Whether you’re navigating ISO EN 13485 or seeking EN ISO 13485 compliance, Vektors is your trusted partner in achieving and maintaining Medical ISO Certification.
Navigating the complex landscape of ISO guidelines for Medical Devices is made simpler with Vektors by your side. Our commitment to excellence is underscored by the integration of ISO 13485 Quality Management System, ensuring your products meet the rigorous standards set by regulatory bodies such as CDSCO Medical Device.
At Vektors, we understand the critical importance of ISO 13845 Certification and Medical Device Registration. Our services cover ISO 13 485, providing a comprehensive approach that includes ISO CDSCO compliance. We specialize in ISO 13458 and offer expertise in the ISO 13845 ISO number, facilitating a smooth and efficient certification process for your organization.
Our integrated approach combines ISO 9001 and ISO 13485, creating a robust Quality Management System (QMS) for Medical Devices. We address the ISO 13485 cost considerations, providing cost-effective solutions without compromising on quality.
Vektors stands as a beacon in the field of ISO Medical Device certification. Our commitment to excellence is further demonstrated through our understanding of 13485 ISO number, ensuring that your products are identified and recognized in the market.
In the ever-evolving landscape of ISO 13485 Medical Devices, Vektors is your dedicated partner, guiding you through the nuances of ISO 13485 version 2016. We seamlessly integrate ISO 9001 13485 standards, offering a comprehensive solution for organizations seeking 13485 ISO 2016 certification.
Choose Vektors for a transformative journey towards ISO 13485:2016 certification, where excellence is not just a goal but a guaranteed outcome. Contact us today to embark on your ISO 13485:2016 certification journey with Vektors. Your path to excellence starts here! Ensure the highest standards in Medical Devices Quality Management.